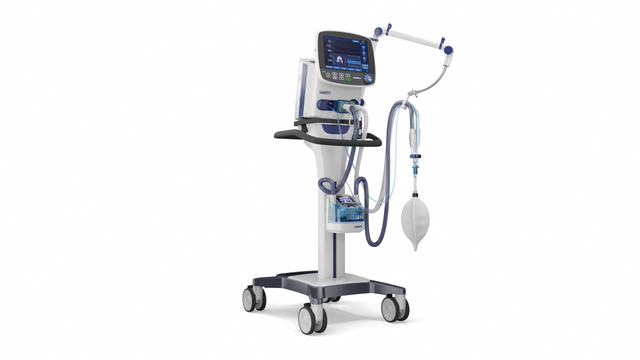
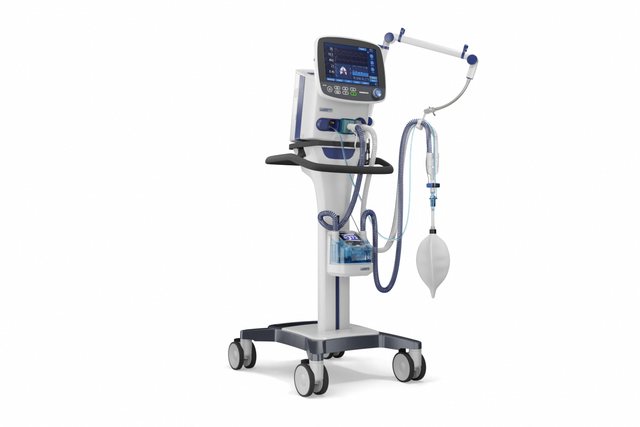
Compact competence. The mobile high-end ventilator

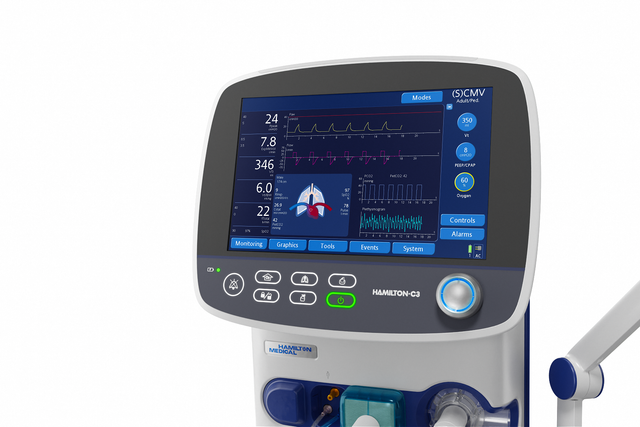
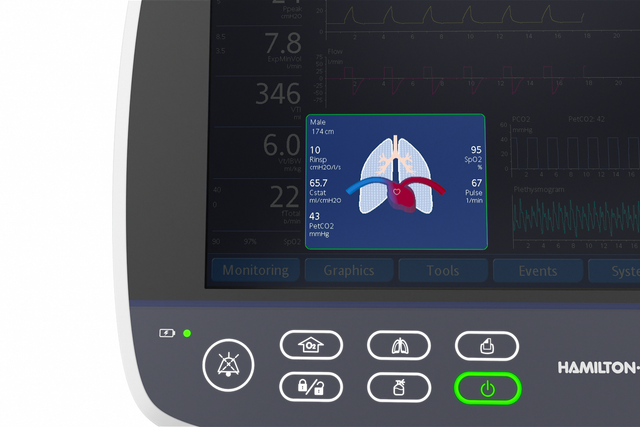
The Dynamic Lung panel displays lung compliance, airway resistance, and patient-triggering in synchrony with the actual breaths.
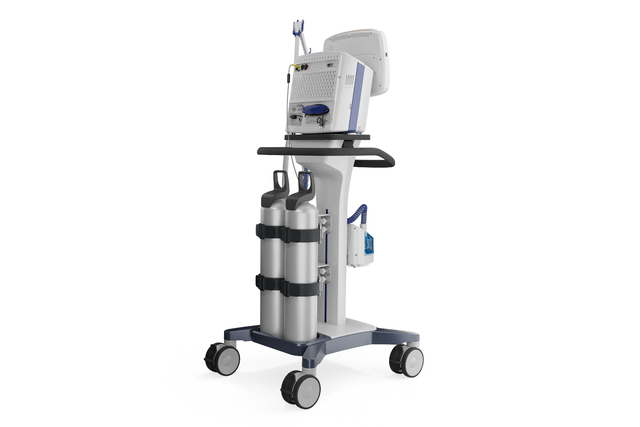
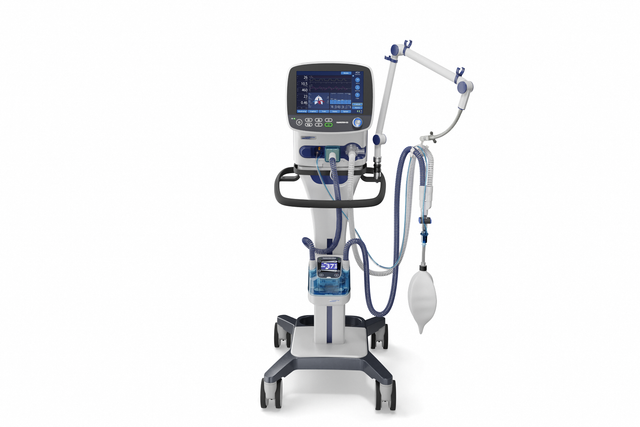

With its powerful turbine, the HAMILTON-C3 is completely independent from a high‑pressure air outlet and external compressors. This gives you maximum mobility so you are free to move around the hospital.
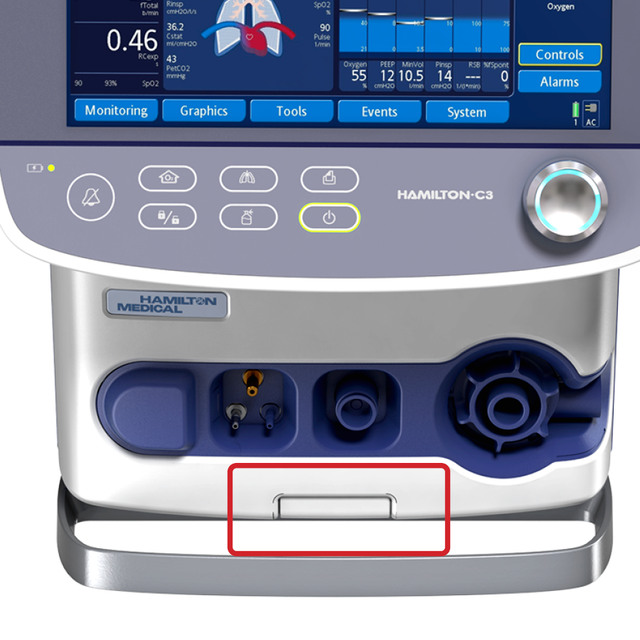
You can attach the HAMILTON-C3 to a trolley, a bed mount or a shelf with ease. No additional tools are needed.
Simply push a button to release the ventilator from the trolley and reattach it with just one click.
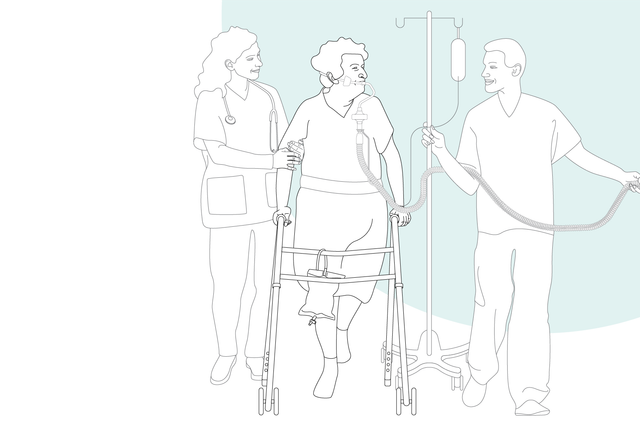
With its high-performance turbine, battery, compact size, and state-of-the-art ventilation modes, the HAMILTON-C3 is also at your patient's side for their first steps out of bed.
Discover the HAMILTON-C3 from every angle and click on the hotspots to learn more.
| Patient groups | Adult/Ped, Neonatal |
|---|---|
| Dimensions (W x D x H) | 310 x 250 x 460 mm (ventilation unit) 560 x 640 x 1460 mm (incl. trolley) |
| Weight | Ventilation unit: 9.5 kg (21 lb) Ventilation unit and trolley: 37 kg (81.6 lb) |
| Monitor size and resolution | 12.1 in (307.3 mm) diagonal 1280 x 800 pixels |
| Detachable monitor | |
| Battery operating time | 2.4 h with one battery 5 h with two batteries |
| Hot-swappable battery | |
| Air supply | Integrated turbine |
| O2 connector | DISS (CGA 1240) or NIST |
| Connectivity | COM port, Nurse call (optional) |
| Loudness | 43 dB in normal operation |
| Volume controlled, flow controlled | |
|---|---|
| Volume targeted, adaptive pressure controlled | |
| Intelligent ventilation | ASV®, INTELLiVENT-ASV® (option) |
| Noninvasive ventilation | |
| High flow | |
| Visualization of lung mechanics (Dynamic Lung) | |
|---|---|
| Visualization of the patient’s ventilator dependence | |
| Esophageal pressure measurement | |
| Capnography | |
| SpO2 monitoring |
| Recruitability assessment and lung recruitment (P/V Tool Pro) | |
|---|---|
| Patient-ventilator synchronization (IntelliSync+) | |
| CPR ventilation | |
| Hamilton Connect Module |
| Remote connection to HAMILTON-H900 humidifier | |
|---|---|
| Integrated IntelliCuff cuff pressure controller | |
| Integrated pneumatic nebulizer | |
| Integrated Aerogen nebulizer | |
| Compatibility with Sedaconda ACD-S anesthetic delivery system |

The HAMILTON-C3 is a completely transportable ventilator. Our fragile patients that go to an operating suite can be maintained on the same ventilator for the whole time during that process.
Neonatal Pediatric Education Coordinator
University Medical Center, Lubbock (TX), USA
The ventilation mode ASV continuously adjusts the respiratory rate, tidal volume, and inspiratory time breath by breath depending on the patient’s lung mechanics and effort - 24 hours a day, from intubation to extubation.
The intelligent ventilation mode INTELLiVENT-ASV continuously controls the ventilation and oxygenation of the patient.
It sets the minute ventilation, PEEP, and Oxygen based on the targets set by the clinician, and on physiologic input from the patient.
You can use the P/V Tool to assess lung recruitability and determine the recruitment strategy.
Additionally, you can use it to perform a sustained inflation recruitment maneuver and measure the increase in lung volume.
The integrated pneumatic nebulizer is fully synchronized with the timing of inspiration and expiration.
An integrated, synchronized Aerogen nebulizer is available as an option (
The delivery of a fine mist of drug aerosol particles helps you reverse bronchospasm, improve ventilation efficiency, and reduce hypercapnia (
High flow nasal cannula therapy (
Proximal flow and CO2 measurement enables our ventilators to perform up-to-date volumetric capnography, which provides an important basis for the assessment of ventilation quality and metabolic activity.
The Vent Status panel displays six parameters related to the patient’s ventilator dependence, including oxygenation, CO2 elimination, and patient activity.
A floating indicator moving up and down within each column shows the current value for a given parameter.
Quick Wean is a feature of the INTELLiVENT-ASV mode that provides continuous dynamic monitoring and control of patient conditions to evaluate the patient’s readiness for extubation.
The automated spontaneous breathing trials (SBTs) are part of the Quick Wean function in the INTELLiVENT-ASV mode and give you the option of conducting fully controlled SBTs.
The Dynamic Lung panel shows you a graphic real-time representation of the following important monitoring data:
The ventilator can display a dynamic loop based on a selected combination of monitored parameters. With the trend function, you can see trending information displayed for the monitoring parameters and time frame of your choice.
The device continually stores the monitored parameters in its memory, even when in Standby.
The SpO2 option offers integrated noninvasive SpO2 measurement with the data displayed conveniently on your ventilator.
We also offer a comprehensive portfolio of SpO2 sensors.
The noninvasive ventilation modes deliver pressure-supported, flow-cycled spontaneous breaths (NIV and NIV-ST mode) and pressure-controlled, time-cycled mandatory breaths (NIV-ST).
Compared to ventilators using compressed air, our turbine-driven ventilators are capable of providing higher peak flow rates. This guarantees optimal performance even with large leaks.
With the nCPAP mode, the patient is supported with a continuous positive airway pressure. In our flow-controlled devices, the desired CPAP value is set via the respiratory gas flow. In order to compensate for any leakage that occurs, e.g. via the mouth or at the nose, the LeakAssist function can be activated. A predefined pressure can then be targeted with additional respiratory gas flow.

Our preassembled breathing circuit sets include the essential consumables to operate the ventilator, conveniently packaged in one single bag.
All our essential consumables are specially developed for Hamilton Medical ventilators with guaranteed manufacturer quality.

To manage ventilation you usually have to set multiple parameters, such as pressure, volume, inspiratory and expiratory triggers, cuff pressure, and more. And each time your patient's condition changes, you have to make one or even several readjustments.
To simplify this process and reduce the knob-turning, we have created a range of solutions:
Adaptive Support Ventilation (ASV) is a ventilation mode that provides continuous adaptation of respiratory rate, tidal volume, and inspiratory time, depending on the patient’s lung mechanics and effort. ASV has been shown to shorten the duration of mechanical ventilation in various patient populations with fewer manual settings (
Our intelligent ventilation mode INTELLiVENT-ASV promotes you from knob-turner to supervisor, reduces the number of manual interactions with the ventilator (
Conventional solutions for cuff pressure management require you to monitor and adjust cuff pressure by hand.
IntelliCuff secures your patient’s airway (

Whenever there is a problem, the ventilator alerts you using the alarm lamp, sound, and message bar.
The on-screen help offers you suggestions on how to resolve the alarm.

We want our ventilator to leave your patient’s side as quickly as possible. That is why we provide you with tools to help you implement your weaning protocol.
These include visual aids and ventilation modes designed to encourage spontaneous breathing.
Our online Academy offers easy-to-follow learning paths to familiarize you with Hamilton Medical products and technologies as quickly as possible.

We are constantly working on further evolving our products. New features are added and existing features improved to ensure you always have access to the latest ventilation technology over your ventilator’s lifetime.


Whether it is in the ICU, in the MRI suite, or during transport, the user interface of all Hamilton Medical ventilators works in the same way.
Our Ventilation Cockpit integrates complex data into intuitive visualizations.
We develop our accessories for the highest possible patient safety and ease of use in mind. Whenever possible, we integrate them with our ventilators to simplify operation of the complete ventilator system.

Our team of Ventilation Geeks is happy to assist you in choosing the perfect ventilator for your clinical care setting and help you meet your therapy goals. Get a personalized price quote or schedule a callback for more information.