
Измерение пищеводного давления (Pes) — это малоинвазивный метод мониторинга, который позволяет определить транспульмональное давление.
Наиболее распространенный способ измерения значения Pes — с помощью наполненного воздухом баллона, встроенного в пищеводный катетер.
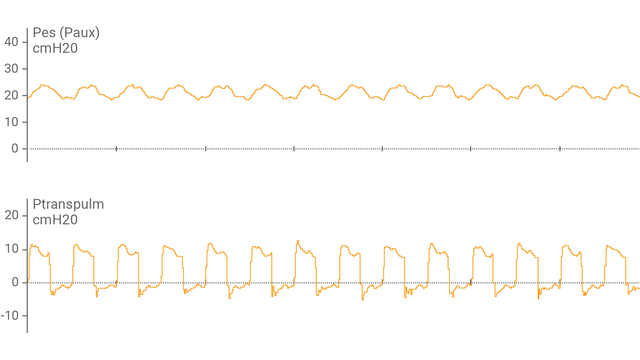
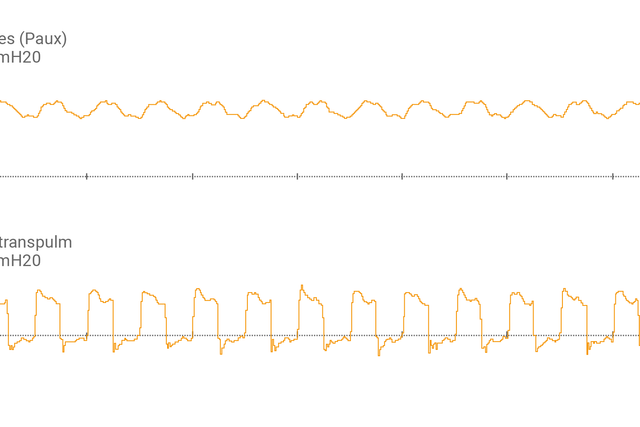
После подсоединения пищеводного баллонного катетера к вспомогательному порту и подтверждения правильности размещения на экране аппарата ИВЛ в виде кривой отобразится пищеводное давление (Pes) и транспульмональное давление (Ptranspulm).
Измерить статическое значение Ptranspulm можно с помощью маневров задержки вдоха и выдоха.
Для оценки возможности раскрытия объема легких и выполнения маневров рекрутмента также можно использовать транспульмональное давление в сочетании с инструментом P/V Tool® Pro.
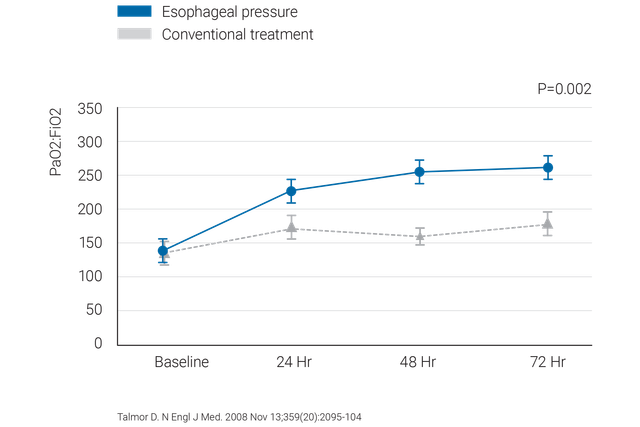


Клинически проверенные рекомендации касательно того, что следует делать и чего следует избегать при измерении пищеводного давления у пациентов с острым респираторным дистресс-синдромом.
Компания Hamilton Medical предлагает пищеводные баллонные катетеры производства компании Cooper Surgical и назогастральные катетеры производства компании NutriVent.
Мониторинг транспульмонального давления – это стандартная функция в аппаратах ИВЛ HAMILTON-C6 и HAMILTON-G5/S1.