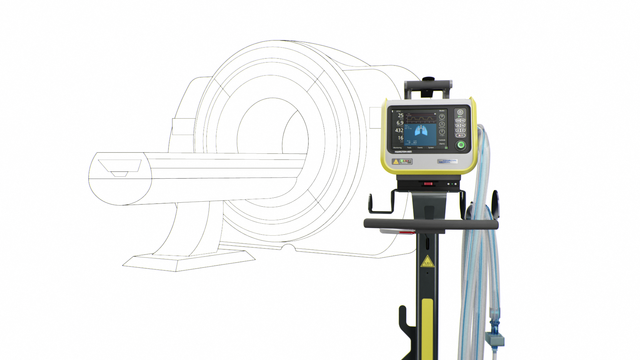
Our MRI specialist. For high-end ventilation during MRI procedures




The TeslaSpy helps you to keep the ventilator at a safe distance from the MRI scanner.

Choose between a range of breathing circuits in different lengths suitable for the MRI suite.

The auto-lock brakes on the trolley automatically lock in place, preventing the ventilator from accidentally rolling toward the MRI scanner.

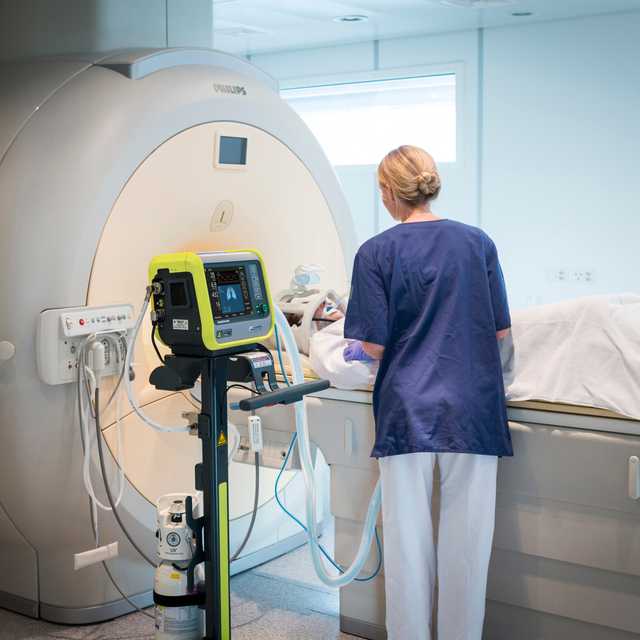
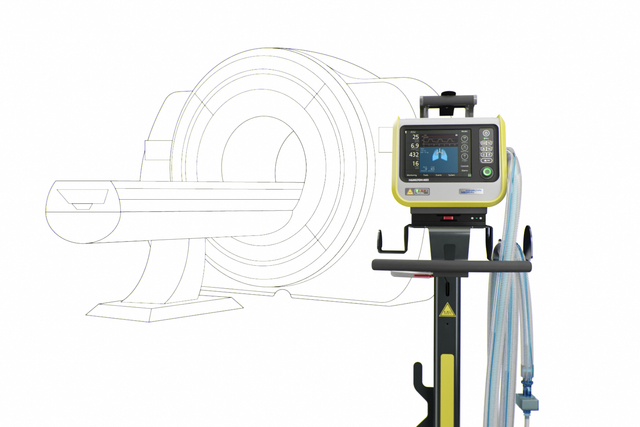
The HAMILTON-MR1 allows you to get close to the MRI scanner, because it is designed to withstand a static magnetic field of up to 50 mT. In comparison to ventilators with weaker shielding from the magnetic field, it gives you more freedom when positioning the device, a wider range of movement, and greater flexibility for your workflow and patient setup.
Closer proximity to the scanner also means you can use shorter breathing circuits. The advantage is their smaller compressible volume, which can contribute to better overall ventilation quality.

The quick‑release button on the transport kit allows you to detach the device from the trolley with just the push of a button. And it reattaches just as easily.
Discover the HAMILTON-MR1 from every angle and click on the hotspots to learn more.
| Patient groups | Adult/Ped, Neonatal |
|---|---|
| Dimensions (W x D x H) | 320 x 220 x 270 mm (ventilation unit) 630 x 630 x 1400 mm (incl. trolley) |
| Weight | 6.8 kg (15 lb) 21 kg (46.2 lb) with trolley |
| Monitor size and resolution | 8.4 in (214 mm) diagonal 640 x 480 pixels |
| Detachable monitor | |
| Battery operating time | 8 h with two batteries |
| Hot-swappable battery | |
| Air supply | Integrated turbine |
| O2 connector | DISS (CGA 1240) or NIST |
| Connectivity | USB port |
| Loudness | 42 dB in normal operation |
| Volume controlled, flow controlled | |
|---|---|
| Volume targeted, adaptive pressure controlled | |
| Intelligent ventilation | ASV® |
| Noninvasive ventilation | |
| High flow | |
| Visualization of lung mechanics (Dynamic Lung) | |
|---|---|
| Visualization of the patient’s ventilator dependence | |
| Esophageal pressure measurement | |
| Capnography | |
| SpO2 monitoring |
| Recruitability assessment and lung recruitment (P/V Tool Pro) | |
|---|---|
| Patient-ventilator synchronization (IntelliSync+) | |
| CPR ventilation | |
| Hamilton Connect Module |
| Remote connection to HAMILTON-H900 humidifier | |
|---|---|
| Integrated IntelliCuff cuff pressure controller | |
| Integrated pneumatic nebulizer | |
| Integrated Aerogen nebulizer | |
| Compatibility with Sedaconda ACD-S anesthetic delivery system |
The ventilation mode ASV continuously adjusts the respiratory rate, tidal volume, and inspiratory time breath by breath depending on the patient’s lung mechanics and effort - 24 hours a day, from intubation to extubation.
The integrated pneumatic nebulizer is fully synchronized with the timing of inspiration and expiration.
An integrated, synchronized Aerogen nebulizer is available as an option (
The delivery of a fine mist of drug aerosol particles helps you reverse bronchospasm, improve ventilation efficiency, and reduce hypercapnia (
The Speak Valve option gives tracheostomized patients a voice, and allows them to swallow even while receiving respiratory support.
The ventilator's monitoring, triggering, and alarm management are adjusted for compatibility with speaking valves in pressure-controlled modes (PCV+, SPONT, PSIMV+).
CPR ventilation adapts the ventilator settings during rescucitation. It supports the CPR workflow with quick access to preconfigurable settings, adequate alarm and trigger adjustment, and CPR-timer display.
The main monitoring parameters and curves relevant to CPR ventilation are also displayed.
The Vent Status panel displays six parameters related to the patient’s ventilator dependence, including oxygenation, CO2 elimination, and patient activity.
A floating indicator moving up and down within each column shows the current value for a given parameter.
The Dynamic Lung panel shows you a graphic real-time representation of the following important monitoring data:
The noninvasive ventilation modes deliver pressure-supported, flow-cycled spontaneous breaths (NIV and NIV-ST mode) and pressure-controlled, time-cycled mandatory breaths (NIV-ST).
Compared to ventilators using compressed air, our turbine-driven ventilators are capable of providing higher peak flow rates. This guarantees optimal performance even with large leaks.
High flow nasal cannula therapy (
The ventilator can display a dynamic loop based on a selected combination of monitored parameters. With the trend function, you can see trending information displayed for the monitoring parameters and time frame of your choice.
The device continually stores the monitored parameters in its memory, even when in Standby.
With the nCPAP mode, the patient is supported with a continuous positive airway pressure. In our flow-controlled devices, the desired CPAP value is set via the respiratory gas flow. In order to compensate for any leakage that occurs, e.g. via the mouth or at the nose, the LeakAssist function can be activated. A predefined pressure can then be targeted with additional respiratory gas flow.

Our preassembled breathing circuit sets include the essential consumables to operate the ventilator, conveniently packaged in one single bag.
All our essential consumables are specially developed for Hamilton Medical ventilators with guaranteed manufacturer quality.

To manage ventilation you usually have to set multiple parameters, such as pressure, volume, inspiratory and expiratory triggers, cuff pressure, and more. And each time your patient's condition changes, you have to make one or even several readjustments.
To simplify this process and reduce the knob-turning, we have created a range of solutions:
Adaptive Support Ventilation (ASV) is a ventilation mode that provides continuous adaptation of respiratory rate, tidal volume, and inspiratory time, depending on the patient’s lung mechanics and effort. ASV has been shown to shorten the duration of mechanical ventilation in various patient populations with fewer manual settings (
Conventional solutions for cuff pressure management require you to monitor and adjust cuff pressure by hand.
IntelliCuff secures your patient’s airway (

Whenever there is a problem, the ventilator alerts you using the alarm lamp, sound, and message bar.
The on-screen help offers you suggestions on how to resolve the alarm.

We want our ventilator to leave your patient’s side as quickly as possible. That is why we provide you with tools to help you implement your weaning protocol.
These include visual aids and ventilation modes designed to encourage spontaneous breathing.
Our online Academy offers easy-to-follow learning paths to familiarize you with Hamilton Medical products and technologies as quickly as possible.

We are constantly working on further evolving our products. New features are added and existing features improved to ensure you always have access to the latest ventilation technology over your ventilator’s lifetime.


Whether it is in the ICU, in the MRI suite, or during transport, the user interface of all Hamilton Medical ventilators works in the same way.
Our Ventilation Cockpit integrates complex data into intuitive visualizations.
We develop our accessories for the highest possible patient safety and ease of use in mind. Whenever possible, we integrate them with our ventilators to simplify operation of the complete ventilator system.

Our team of Ventilation Geeks is happy to assist you in choosing the perfect ventilator for your clinical care setting and help you meet your therapy goals. Get a personalized price quote or schedule a callback for more information.